|
 |
 |
Volume
5 - Issue 07
JULY 2007 |
|
By Prof. G Venkataraman
Loving Sai Ram and greetings again. I am sorry there was a break in presenting this series but then you know there was a good reason for it! In my last article, I dealt with Black Holes, prior to which I discussed White Dwarf and Super Nova explosions. When I was away in Kodai during May, I read in the papers that astronomers have discovered an explosion that appears to be the “mother of them all”.
SN 2006gy occurred in a distant galaxy [NGC 1260] approximately 238 million light years away. Therefore, due to the time it took light from the supernova to reach the Earth, the event occurred about 238 million years ago. Preliminary indications are that it was an unusually high-energy supernova of a very large star, around 150 solar masses. That is a real wow! The energy released by the explosion has been estimated as ten times more than the typical supernova explosion. The supernova's light brightened for about 70 days after discovery, until roughly the beginning of December 2006, and has been slowly decreasing since then. So much for the new excitement; let me now go back to the main trend of my earlier instalments. You will recall that thus far we have had 1) a look at what makes up the Universe, 2) the birth and death of different types of stars and the types of corpses they lead to. There are many more details I could give about the Universe and its makeup but right now, that would take us too far off our main course.So, at this point I would like to pause and pose the question that many have asked themselves before, which is: “Where did it all begin and what was the baby Universe like? How did it grow thereafter to its present vast size?” Since the Universe is a physical entity, it is obvious that a large part of the answer to the question just posed would have to come from Physics. So let us take a small detour and delve a bit into Physics. Physics to Understand Physical Universe
Journey into the Microcosm Let us try to understand this last remark. In astronomy, we deal with very large scales of distances that are measured in light years. As for the time scale, a few hundred thousand years is no big deal, considering that stars live for millions of years. Come down to earth and in normal life, distances we encounter range from kilometres to millimetres and may be a bit less, like the thickness of a hair. As for time, it ranges from years to minutes to seconds and may be fractions of a second like a millisecond or one thousandth of a second. Looking through a microscope, we can begin to see bacteria and things like that which are much smaller [and therefore require a microscope].
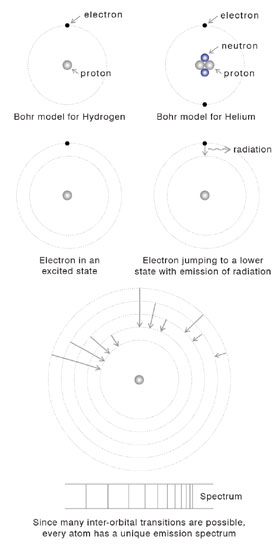
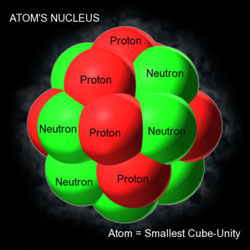
OK, we are now down to the level of atoms, whose dimension is typically 10-8 cm or one hundredth of one millionth of a centimetre – that is small, is it not? Well, wait and soon you would encounter distances that are still smaller. Once physicists [and of course chemists who came first!] accepted the atomic theory of matter, it was universally realised that though matter might appear continues to us in our daily experience, it is pretty grainy when one comes down to the level of atoms. As far as time scales are concerned, time intervals like 10-12 seconds [i.e., one millionth of one millionth of a second!] are what one usually deals with. Atoms write their signatures through what are called spectral lines. The word spectral lines need not stump you. When energy is pumped into an atom [there are many ways of doing this], it becomes excited. This excitation energy is subsequently got rid off by the atom and it does this usually be emitting light at specific wavelengths. Spectral lines are just signatures of the light emitted by an atom while de-exciting. Study of atomic spectra is thus of vital importance in many branches of modern activity, ranging from basic physics and chemistry all the way to forensic science. Until 1900, people imagined atoms were like hard billiard balls, but when J. J Thomson of Cambridge discovered around that time that he could chip an atom and squeeze electrons out of them, people realised that atoms had their own internal structure. It seemed as if atoms were made up of electrons that were negatively charged and that positive charge was tucked in somewhere within the atom. Around 1910 or so, Niels Bohr of Denmark tried to imagine a model for atomic structure and develop a theory for spectral lines emitted by atoms when they emit radiation. Where structure was concerned, Bohr said that the atom was like a mini solar system with a tiny atomic nucleus at the centre [rather like our Sun] and electrons going round it in circular orbits. In the Bohr model, all the positive charge of the atom was locked up in a tiny region called the atomic nucleus or just nucleus for short. The electrons that carried the negative charge whirled around the nucleus like the planets. Clearly, Bohr was mimicking the solar system and there was nothing wrong in that. Scientists often make models, based on examples presented by Nature itself.
After imagining all this, Bohr then wanted to describe the motion of the electrons, calculate their energies and so forth. For doing this, one needed a theory of mechanics and all that one had then was Newton’s theory for describing the behaviour of moving bodies, [modified by Einstein to include relativity, if the objects moved real fast, that is to say at speeds close to that of light].
Birth of Quantum Mechanics Bohr did just that and in 1913, came out with his now famous Bohr model of the atom, and it was an instant breakthrough. Sure, Bohr’s model left a lot of things unexplained, but it certainly was a big leap forward. One thing led to another and by 1928, one had what is now called quantum mechanics – which has since become the standard grammar of physics at the microscopic level. Physicists said, “Listen, whatever it is that you deal with at microscopic distances, you better use quantum mechanics to do that.” So where physicists were concerned, quantum mechanics became their new Veda, and the Rishis who discovered this were Erwin Schordinger of Austria, Werner Heisenberg of Germany and Paul Dirac of England. I should not forget Prince de Broglie of France who in 1924, preceding the trio just mentioned, made the radical suggestion that if light had a dual nature representing its quantum nature, then so does matter! In other words, not only radiation but matter too was quantum in nature, an aspect that reveals itself mostly on small scales of distance. That is why it was missed till the twentieth century. OK, so by 1930, physicists had a mechanics for describing the behaviour of moving charges right down to the quantum level. There was still some dust around that had to be cleared but physicists developed new interests and started becoming curious about the nature of the atomic nucleus. This opened up an entirely new vista that we now call nuclear physics. Thus, in a manner of speaking, the period 1930 to 1939 saw the rapid growth of nuclear physics, with quantum electrodynamics as developed by Dirac in 1928, put aside for the moment. Some people realised that Dirac’s quantum electrodynamics was incomplete but no one knew how to fix it. Besides, nuclear physics seemed so much more inviting! 1939, World War II broke out. America was not in the war yet, but Europe was in the thick of it. A good many European scientists of Jewish origin had fled from Germany and other countries under Hitler’s domination, and many of them took refuge in America. In 1939, Hahn and Meitner in Germany had discovered nuclear fission and while nuclear fission was good physics, physicists in France and European scientists who had taken refuge in America realised that fission could be made the basis for an entirely new type of bomb with unimaginable power of devastation. That led to the secret atomic bomb project culminating in the development of the atomic bomb by America in early 1945. Meanwhile of course, America itself became involved in the war, when the Japanese attacked Pearl Harbour. In August 1945, two atoms bombs were dropped over Japan, and the destruction they produced left everyone stunned. Japan surrendered quickly and the war was over.
Return of Quantum Electrodynamics In America, physicists eagerly returned to their academic ivory tower and suddenly, quantum electrodynamics became very attractive. Some very precise experiments were performed, and those results simply could not be explained using the Dirac version of quantum electrodynamics [QED] which till then was thought to be the end all. A Version 2.0 was needed but it was not easy to find. However, three young people, Feynman and Schwinger in America and Tomanaga in Japan independently discovered this version, and it made great waves. This new, improved version [that almost sounds like a toothpaste ad but this version of QED deserved all the adjectives one could use!] was put to one test after another and it explained experimental results with hitherto unimaginable precision. I mean they would do a very precise experiment, yielding a result accurate to seven or eight places of decimals. QED calculations would give the same answer. In fact, they would give to more decimal places, forcing experimenters to improve their accuracy. So for some time, it was experiment and theory each trying to outdo the other. QED was so successful, that Feynman described it a Jewel of Physics. After a while, physicists said, “OK, QED is great but let us move on and turn our attention to the mysteries inside the nucleus more carefully.” Thus from 1954 or so to say 1970, a lot of attention was given to nuclear physics. The first question asked was: “What on earth is the nucleus exactly?” Bohr said it is a small entity with positive charge and Rutherford in Cambridge had not only determined the size to be around 10-12 cm or so, but also figured out that the nucleus was made up of protons and neutrons. From then on, it was one question after another, almost endlessly. When one question got answered, another popped up – that is the way it is in science which is why many head for science; it gives a high non-stop, without drugs that is! So where was Physics, say around the seventies? That can be summarised as follows:
Mystifying Story of Matter I am sure all this must be rather mystifying and so let me add several explanatory notes and comments, starting with the so-called building blocks. Actually, here the views have changed quite a bit over the last few centuries. At the time of Newton when the atomic theory of matter simply did not exist, there was no question of building blocks of matter. This has an important implication. Let us suppose that matter is continuous. Take a piece of matter like iron rod, say 1 cm diameter and 10 centimetres long. Cut it in the middle. We would then get two pieces each 5 cm long. Take one piece and cut that; this time the pieces would be 2.5 cm. Keep doing this on and on. As you do so, the pieces would get thinner and thinner and thinner. In principle, there should be no limit, because since matter is supposed to be continuous, the slices cut can be indefinitely thin. Suppose now matter is made up of atoms that are like infinitely hard spheres; that is to say an individual atom simply cannot be cut. If the above slicing process is gone through, then in this case, one would eventually come to a slice that is one atom layer thick; that is the limit and one cannot under the assumption of an indestructible atom, go any further.
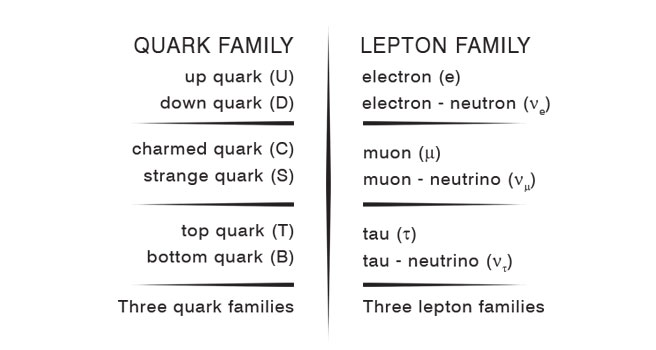
Unearthing the Twosome - Leptons and Quarks It was a hard grind finding all this, but by 1950, physics had become a big arena with hundreds if not thousands of physicists working all over the world. So a lot of information started piling up, rather quickly, one must mention, and by 1970 or so, one could sort of say: “Well you know what? We now can be sure what the basic building blocks of matter are, and list them.” This was done, and it was found that the building blocks were of two fundamental categories; one of them was called leptons and the other was called quarks. Leptons meant light particles and the electron was identified as a member of the lepton family. As for quarks, it is combinations of them that make up the neutron and the proton. In other words, one can now really say: “I don’t care if it is an ant or the Statue of Liberty. It is all made up of electrons and quarks – that’s it!”
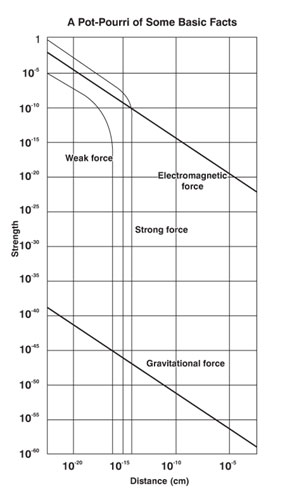
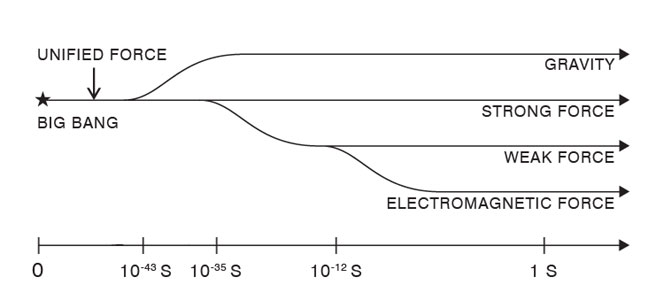
May sound simple but in practice, as one goes deeper and deeper into the atom, both theory and experiment become quite challenging and difficult. This is the game for the big boys! But a lot of big boys did get in there and did wonders; no wonder many of them picked up the coved Noble! The Fundamental Forces So much for the building blocks. What about the forces via which this basic building blocks operate? In the beginning, discovery of forces came about quite independently; it is only after say 1950 that the study of the basic forces and the building blocks started going hand in hand. Well, how many different kinds of basic forces do we have and what do we know about them? The basic forces have already been enumerated; there are four of them. Historically, the first to be discovered was the gravitational force. As you perhaps know, Newton made that discovery, and it was an important moment in history. Newton said matter attracts matter, always. It does not matter if the piece of matter is big or small; a big stone thrown upwards is attracted by the earth and falls; so does a small stone. You may say a feather does not fall easily as compared to say a ball with the same mass. Well, the force of attraction depends only on 1) the masses of the two objects concerned and 2) the distance separating them. Feather falls softly because of the atmosphere, which acts like a cushion. Take that same feather to the Moon [where there is no air] and drop it there; you will find the feather and the ball with the same mass would fall at the same rate. From the gravitational force, we turn next to the electromagnetic force which operates between any two charged particles. Thus, since the electron and the proton both carry electrical charge, there is between the two, an electromagnetic force as well as a gravitational force. However, the gravitational force acting between the two is so small [about one part in 1 followed by may be 40 zeros!], that the gravitational force between the electron and the proton is completely ignored, as was done by Niels Bohr when he developed the theory of the hydrogen atom. In contrast to the gravitational force, information about the electromagnetic force came in bits and pieces due to the investigation of many physicists over a period of time. But in due course, one had a complete understanding of it. What now about the so-called weak and the strong force? Where do they operate and why are they so named? The answers to all those questions is simple. Firstly, both these forces, the weak as well the strong, operate only when the distances between the concerned particles are very small? How small is small? Around 10-13 cm and less! In other words, consideration of the weak and the strong force play an important role in nuclear physics and the physics of elementary particles in general, that is of leptons and quarks. The discovery of the weak force was made during intensive studies on radioactivity, and the man who formally introduced it was Enrico Fermi [then of Italy], later to build the world’s first atomic reactor as also the first atom bomb! To put it in perspective I should perhaps say that a study of leptons is impossible without bringing the weak force into the picture.
You are perhaps wondering: “What is this man up to? We are supposed to be talking about Infinity, which obviously must be something unimaginably huge; and here is all this chat about the infinitesimal! Has he gone bananas?” I assure I have not! You see God, the quintessence of Infinity, reveals Himself everywhere, from the infinitesimal to the Infinite. Right now, I am trying to take you to the microcosm of the physical Universe just to highlight how even in respect of the Universe, the micro and the macrocosm are intimately intertwined. With that caveat, let me proceed. Two questions arise at this point: “Why only four fundamental forces? Why not more?” This is question number one. Second question: “Are these forces completely unrelated to each other or is there some connection? If so, what is that connection?” These are the two questions that would concern us next. Let us start with the first of the two questions above. After a lot of intense debate, physicists are generally agreed that there are only four fundamental forces and no more. For a while there was a flutter about a fifth force but that whisper has since died down. As regards the second question, the consensus is: “When the Universe was born, there was actually one Universal Force. But almost within a second after birth, this one Universal Primordial Force split into four, to facilitate the further evolution of the Universe. The history of the Universe from that moment of separation has been governed by four and only four fundamental forces.
A small corollary. We often find physicists and chemists talking of all kinds of forces like the forces of surface tension, elastic forces and so on. All these are derived forces that operate within a certain context; they are not fundamental in the sense they do not determine the way leptons and quarks behave. Please keep that in mind. The derived forces are not unphysical but they are relevant only in limited context; our concern is with the Universe as a whole and in that context only the four forces I have listed above are pertinent. Hope that is clear. Understanding ‘the small’ is Unavoidable You might still say: “All this is fine, but I am still not clear why this digression from the Universe into nuclear physics, particle physics and all that.” Point well taken, and maybe I should say something to address that concern of yours before taking leave. You see, the reason why I am currently insisting on talking about all this is to give you some idea of physics at very small distances. When I say very small distances, I mean incredibly small distances like 10-40 cm and things like that!
You may say: “Hey, wait a minute! Why at all do we want to know about the Baby Universe? In any case, I am not too sure I would believe all that you might say; it was so long ago!” Good point, but you see, today’s astrophysicists are so smart, if you tell them, “Look the Baby Universe was like so,” they would reply, “Is that so? We will go out and check and get back.” They then go out and look into the sky – and they have amazing tools now to do that, and when they are finished, they would report, “Mister, we have bad news for you. Your hypothesis about the Baby Universe is not quite ok. These are things we found out there. If the Universe had been like what you say then, it follows it should have been like this now; but it ain’t!” Thanks to this sort of constant chat back and forth, our picture of the Universe has become slowly sharpened all the way back say about 10-10 seconds. This has happened in just about twenty years or so; amazing it is not? Of course the story is far from complete because that last bit, from 10-10 to say 10-50 seconds is going to be very, very tough. But people are getting smarter and smarter all the time, and may be in fifty years time we would know about a still smaller Baby than we do now, that is if we have not screwed up the world further with conflict, wars, global warming and all that! I should call a halt, but before I do so, I want you to reflect on the following:
God does not care what man does. However, He does care about helping man and guiding man. He comes down to advice and act as a guidepost. As Swami says, the guidepost tells the traveller in which direction he or she should go. It does not go with the traveller! Think about it! All the best till we meet again! Jai Sai Ram.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| You can write to us at : h2h@radiosai.org |
Vol 5 Issue 07 - JULY 2007
|
Best viewed in Internet Explorer - 1024 x 768 resolution. |